| Development of microfluidic tools to study aging in budding yeast
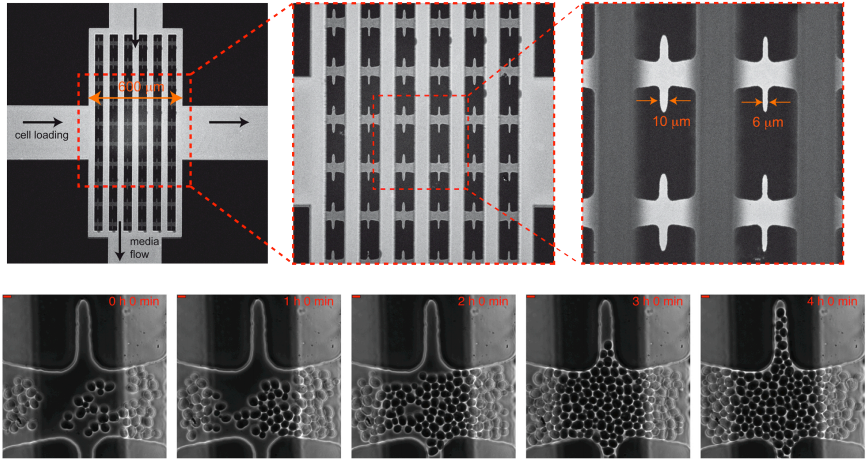
Under the supervision of Gilles Charvin, Single Cell Biophysics – IGBMC, Strasbourg, France // April-August 2011 Budding yeast cells divide asymmetrically, with each mother cell producing a limited number (∼25) of smaller daughter cells before it enters senescence and eventually dies. Remarkably, daughters of aged mother cells recover a full replicative lifespan (RLS; total number of cell divisions), which implies that a rejuvenation mechanism exists to avert clonal senescence. Although a large number of mutations are known to modulate RLS, the precise causes of senescence and rejuvenation, and thus their heritability, are poorly understood. We developed a microfluidic device to monitor the dynamics of cell division and follow a single mother cell from birth to death in order to better understand mechanisms of senescence and rejuvenation. Picture (right): Microfluidic micrometric device to trap mother cells from birth to death named CLiC. On the bottom, cells proliferate and colonize the CLiC device. Scale bars: 5µm. |
 |
Laser ablations in pupae Drosophila wing
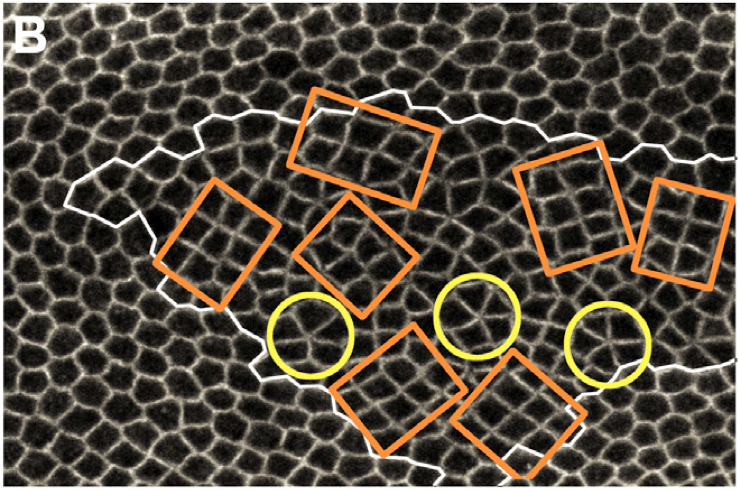
Under the supervision of Yohanns Bellaiche, Genetics and Development Biology, UMR934 Curie Institute, Paris, France // Jan-March 2011 Planar cell rearrangements control epithelial tissue morphogenesis and cellular pattern formation. They lead to the formation of new junctions whose length and stability determine the cellular pattern of tissues. Here, we show that during Drosophila wing development the loss of the tumor suppressor PTEN disrupts cell rearrangements by preventing the lengthening of newly formed junctions that become unstable and keep on rearranging. We demonstrate that the failure to lengthen and to stabilize is caused by the lack of a decrease of Myosin II and Rho-kinase concentration at the newly formed junctions. This defect results in a heterogeneous cortical contractility at cell junctions that disrupts regular hexagonal pattern formation. By identifying PTEN as a specific regulator of junction lengthening and stability, our results uncover how a homogenous distribution of cortical contractility along the cell cortex is restored during cell rearrangement to control the formation of epithelial cellular pattern. Picture (right): Drosophila wing ephithelium. The tumor suppressor PTEN was disrupted in cells inside the white contour. Such mutant cells rearrange into chocolate bars (cobblestone – orange contours) or rosettes (yellow contours). During this internship, I probed the tension of cellular junctions in wild type cells (outside the white contour) and PTEN mutant cells and established that there is no difference of tension in PTEN mutant cells and wild type cells. |
 |
Biomimetics of diatoms
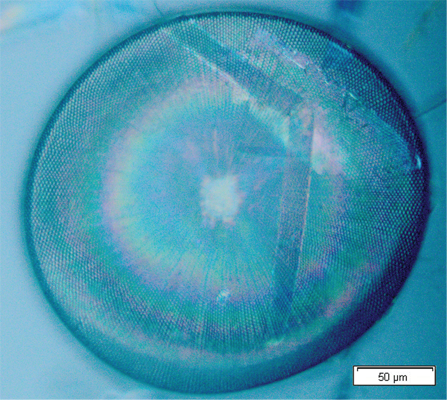
Under the supervision of Christian Grillet, CUDOS, University of Sydney, Australia // May-July 2010 Diatoms are algae whose silica shells could have a periodic pattern. Thus, they theoretically behave as man-made photonic crystals. The purpose of this internship was to carry out preliminary tools from scratch in order to characterize the diatoms’ nanostructures, measure the diatoms’ photonic properties and compare those experimental results with numerical predictions. The species Coscinodiscus walesii has been chosen for this study. I characterized diatom’s structure by analizing optical microscopy and Scanning Electron Microscopy images.I performed simulations with the numerical commercial software RSoft and got the band structure due to the periodic pattern. Finally I built two different setups to measure diatoms’ photonic properties. One using a tapered optical fibre to test evanescent coupling. In agreement with simulations no coupling has been observed. The other using a normal incident laser beam to observe whether the diatom behaves like a micro-lens. Picture (right): Single diatom Coscinodiscus walesii observed under the microscope. The hexagonal pattern of silica holes makes this marin algae behaving like a micro-lens! |
 |
Measure of karyoplasmic ratio by deconvolution microscopy
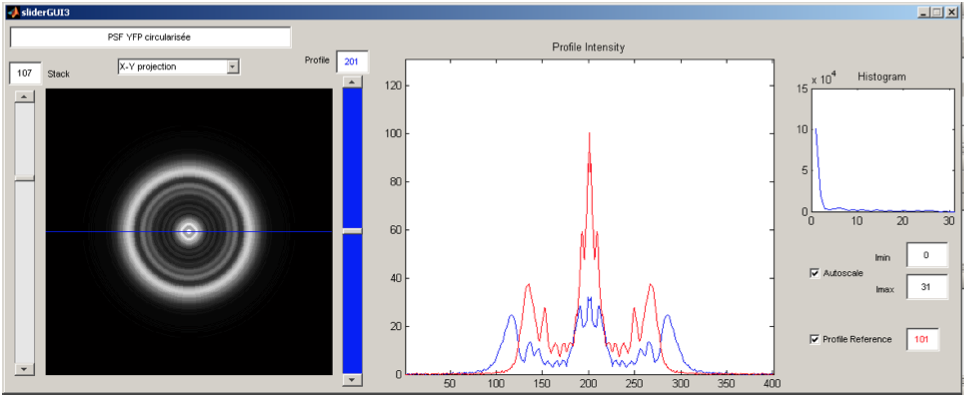
Under the supervision of Gilles Charvin, Single Cell Biophysics – ENS Lyon, France // June-July 2009 The mechanisms underlying the cell size control in yeast and its organelles are a major subject of studies in the field of cellular biology, but remain for the major part unknown. However controlling these sizes accurately is essential to the cell cycle. During this 2-month internship, the karyoplasmic ratio was evaluated through direct measurement of cell and nuclear volumes using a home-made deconvolution algorithm applied to 3D wide-field fluorescence microscopy. The measurements were performed on single cells allowing for a time lapse analysis of the cell cycle. Picture (right): Graphical User Interface (GUI) created to analyze a Point Spread Function (PSF). |
 |



